|
The components of the electron transport chain in eukaryotes are located in the inner mitochondrial membrane. These components are organized into four complexes. Each complex consists of several proteins and prosthetic groups.
Complex I:
复合物I,也称为NADH脱氢酶复合物。复合物I催化由NADH电子向UQ(辅酶Q,辅酶)转移。络合物I是在内膜的最大蛋白质组分,它由至少43多肽为850 kD的总质量。它包含黄素单核苷酸(FMN,仅由不存在所述的AMP组不同于FAD氧化还原辅基)的一个分子。此外,为FMN的一个分子,复合物包含七个铁硫中心。铁硫中心,它可以由复合有相等数量的硫化物离子的两个或四个铁原子,调解1-电子转移反应。两种最常见的类型指定的[2Fe-2S]和[4FE-4S]簇。含有铁硫中心的蛋白质总是被称为非血红素铁蛋白。Complex I, also known as NADH dehydrogenase complex. Complex I catalyze the transfer of electrons from NADH to UQ (coenzyme Q, Ubiquinone). Complex I is the largest protein component in the inner membrane, it composed of at least 43 polypeptides with total mass of 850 kD. It contains one molecule of flavin mononucleotide (FMN, A redox prosthetic group that differs from FAD only by the absence of the AMP group). In addition, to one molecule of FMN, the complex contains seven iron-sulfur centers. Iron-sulfur centers, which may consist of two or four iron atoms complexed with an equal number of sulphide ions, mediate 1-electron transfer reaction. The two most common types designated [2Fe-2S] and [4Fe-4S] clusters. Proteins that contain iron-sulfur centers are always referred to as nonheme iron proteins.
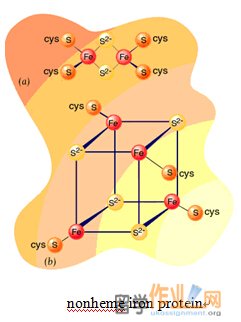
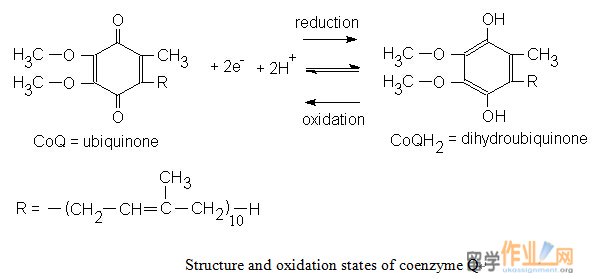
铁硫簇可经历单电子氧化及还原。所有的铁 - 硫簇的氧化还原状态的一个正式起诉他们的铁原子的数量不同而不管。这是因为,在每个簇Fe原子形成共轭体系,从而可以具有2和3的值之间的氧化状态。 FMN是紧密结合的蛋白质,而辅酶Q具有疏水性尾部,使得它可溶于线粒体内膜的脂质双层。Iron- sulphur clusters can undergo one-electron oxidation and reduction. The oxidized and reduced states of all iron-sulfur clusters differ by one formal charge regardless of their number of Fe atoms. This is because the Fe atoms in each cluster form a conjugated system and thus can have oxidation states between +2 and +3 values. FMN is tightly bound to proteins while coenzyme Q has a hydrophobic tail that makes it soluble in the inner mitochondrial membrane’s lipid bilayer.
NADH reduces FMN to FMNH¬2. Electron was then transferred from FMNH2 to an iron-sulfur center, 1 electron at a time. After transfer from one iron-sulfur center to another, the electrons are eventually donated to CoQ.
Inhibitor of complex I:
NADH减少FMN到FMNH¬2。电子然后从FMNH2转移到铁硫中心,1电子的时间。从一个铁硫中心转移到另一个之后,电子被最终捐赠给辅酶Q。The best-known inhibitor of complex I is rotenone. Rotenone binds to the ubiquinone binding site of Complex I as well as piericidin. Potent A is another potent inhibitor with a close structural homologue to ubiquinone.
Complex II:
Succinate dehydrogenase or succinate-coenzyme Q reductase (SQR) or Complex II is an enzyme complex, bound to the inner mitochondrial membrane. It consists primarily of the citric acid cycle enzyme succinate dehydrogenase and two iron-sulfur proteins. Complex II mediate the transfer of electrons from succinate to CoQ. Its redox groups include succinyl dehydrogenase’s covalently bound FAD to which electrons are initially passed, one [4Fe-4S] cluster, two [2Fe-2S] clusters, and one cytochrome b560 (cytochrome are electron transport heme proteins). The oxidation site for succinate is located on the larger of the iron-sulfur proteins. Other flavoproteins also donate electrons to CoQ.
The free energy for electron transfer from succinate CoQ is insufficient to drive ATP synthesis. The complex is nevertheless important because it allows relatively high-potential electrons to enter the electron transport chain by passing complex I. Both complex I and complex II accomplish the same result: the transfer of electron to CoQ from reduced substrates (NADH or succinate). CoQ, which diffuses in the lipid bilayer among the respiratory complexes, therefore serves as a sort of collection point for electrons. Glycerol-3-phosphate dehydrogenase, an enzyme located on outer face of inner mitochondrial membrane, transfers electrons from cytoplasmic NADH to the electron transport chain. In the other way, Acyl-CoA dehydrogenase, the first enzyme in fatty acid oxidation transfer electrons to CoQ from the matrix side of the inner membrane.
Inhibitors of complex II:
There are two distinct classes of inhibitors of complex II. Those that bind in the succinate pocket and those that binds in the ubiquinone pocket. Ubiquinone type inhibitors include carboxin and thenoyltrifluoroacetone. Succinate-analogue inhibiitors include the synthetic compound malonate as well as the TCA cycle intermediates, malate and oxaloacetate. Indeed, oxaloacetate is one of the most potent inhibitors of Complex II.
Complex III:
Complex III transfers electrons from reduced coenzyme Q (UQH2) to cytochrome c. It contains two b-type cytochromes, one cytochrome c1 (cyt c1), and one [2Fe-2S] cluster (known as Rieske center), complex III is sometimes referred to as the cytochrome bc1 complex. The cytochromes are a series of electron transport proteins that contain a heme prosthetic group similar to those found in hemoglobin and myoglobin.
Complex III functions to permit one molecule of CoQH2, a two-electron carrier, to reduce two molecules of cytochrome c, a one- electron carrier. This occurs by the flow of electrons from CoQH2 to cytochrome c1 and to cytochrome b. This process is so called Q cycle, which permits complex III to pump protons from the matrix to the intermembrane space.
The overall cycle is actually two cycles, in first reaction, coenzyme QH2 is supplied by complex I on the matrix side of the membrane. Then, QH2 diffuses to the cytosolic side of the membrane, where it binds in the Qo side on the cytochrome b subunir of complex III. Next, QH2 reduces the Rieske iron-sulfur protein (ISP), forming CoQ – semiquenone and releasing 2H+. The ISP goes on to reduce heme c1. CoQ – reduces heme bL to form coenzyme Q. Q in cycle 1 and cycle 2 diffuses to the matrix side, where it binds in the Qi site on cytochrome b. Heme bL reduces heme bH and Q is reduced to CoQ- by heme bH. In second cycle, CoQ- bound in the Qi site is reduced to CoQH2 by heme bH. The net reaction is the transfer of two electrons from CoQH2 to cytochrome c1 and the translocation of four protons from the matrix to the intermembrane space.
The reaction of the first cycle:
CoOH2 + cytochrome c1 (Fe3+) CoQ- + cytochrome c1 (Fe2+) + 2H+ (cytosolic)
The reaction of the second cycle:
CoOH2 + CoQ- + cytochrome c1 (Fe3+) + 2H+ (mitochondrial)
CoQ + CoQH2 + cytochrome c1 (Fe2+) + 2H+ (cytosolic)
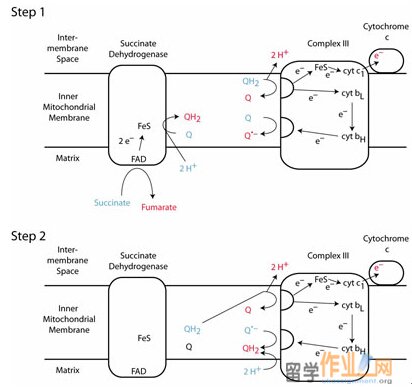
The circuitous route of electron transfer in complex III is tied to the ability of coenzyme Q to diffuse within the hydrophobic core of the membrane in order to bind to both the Qo and Qi side. When CoOH2 is oxidized, two reduced cytochrome c molecules and four protons appear on the outer side of the membrane. Proton transport by the Q cycle thus differs from the proton-pumping mechanism of complex I and IV. In Q cycle, a redox center itself (CoQ) is the proton carrier.
Cytochrome c:
Cytochrome c is a soluble electron carrier. The electrons that flow to cytochrome c1 are transferred to cytochrome c, which, unlike the other cytochromes of the respiratory electron transport cgain, is a peripheral membrane protein. It shuttles electron between complex III and IV on the outer surface of the inner mitochondirial membrane. Several Lys residues in cytochrome c lie in a ring around the exposed edge of its otherwise buried heme group. These residues constitude a binding site that was identified by differential labeling: Treatment of cytochrome c with acetic anhydride in the presence and absence of cytochrome c1 demonstrated that cytochrome c1 completely shields these cytochrome c Lys residues. The reactivities of other cytochrome c Lys residues that are distant from the exposed heme edge are unaffected by the presence of cytochrome c1. Nearly identical results were obtained when cytochrome c1 was replaces by cytochrome c oxidase. Evidently, both of these proteins have negatively charged sites that are complementary to the ring of positively charged Lys residues on cytochrome c. Such ion pairing interactions probably align redox groups for optimal electron transfer.
Inhibitors of complex III:
The inhibitor of complex II is antimycin that binds to the Qi site and inhibits the transfer of electrons in Complex III from heme bH to oxidized Q (Qi site inhibitor).
Complex IV: Cytochrome oxidase is a protein complex that catalyzes the4-electron reduction of O2 to form H2O.
4 Cytochrome c (Fe2+) + 4H+ O2 4 cytochrome c (Fe3+) + 2H2O#p#分页标题#e#
The membrane spanning complex in mammals may contain between six and thirteen subunits, depending on species. The core of complex IV consists of its three largest and most hydrophobic subunits, I, II, and III, which are encoded by mitochondrial DNA. A concave area on the surface of the protein that faces the intermembrane space contains numerous acidic amino acids that can potentially interact with the ring of Lys residues in cytochrome c, the electron donor for complex IV.
Complex IV contains four redox centers: cytochrome a, cytochrome a3, a copper atom known as CuB, and a pair of copper atom known as the CuA center. In addition, there is a Mg2+ ion and a Zn2+ ion. Its two copper ions are bridged by two sulfur atoms of Cys residues. The Mg2+ ion may participate in electron transfer or stabilize the arrangement of redox centers; Zn2+ is far from the redox centers, almost certainly plays a structural rather than a catalytic role.
The reduction of O2 to 2 H2O by cytochrome oxidase takes place at the cytochrome a3-CuB binuclear complex. This reaction, which goes to completion in ~1 ms at room temperature, involves four consecutive one-electron transfers from the CuA and cytochrome a sites and occurs as follows:
1. The binuclear Fe(III)a3-Cu(II)B complex is reduced, by two one-electron transfers from cytochrome c via cytochrome a and the CuA center, to its Fe(II)a3¬-Cu(I)B form.
2. O2 binds to the reduced binuclear complex so as to bridge its Fe(II) and Cu(I) atoms.
3. Internal electron redistribution rapidly yields the stable peroxy adduct Fe(III)-O—O-Cu(II).
4. A further one-electron transfer together with the acquisition of a proton converts the adduct to Fe(III)-O—OH Cu(I)
5. The acquisition of a second proton and an electronic rearrangement results in Fe(IV)=O2- H2O-Cu(II).
6. The fourth one-electron transfer together with a proton rearrangement rhen yields Fe(III)-OH- -HO-Cu(II).
7. Finallly the acquisition of two more protons yields 2H2O together with the Fe(III)a3-Cu(II)B complex, thereby completing the cycle.
Four protons are consumed in the reduction of O2 by cytochrome oxidase. These protons originate in the mitochondrial matrix and probably reach the heme a3-CuB complex via a hydrogen bond network that includes groups on the protein as well as water molecules that occupy cavities in the protein structure. The product of the reaction, water, exists the enzyme via a hydrophilic channel strectching from heme a3, between subunits I and II, to the cytosolic surface of the enzyme. A conformational change that occurs on reduction of the Fe(III)a3-Cu(II)B complex may open an O2 channel, presumably lined with hydrophobic groups, possibly including membrane lipids that bind tightly to the protein.
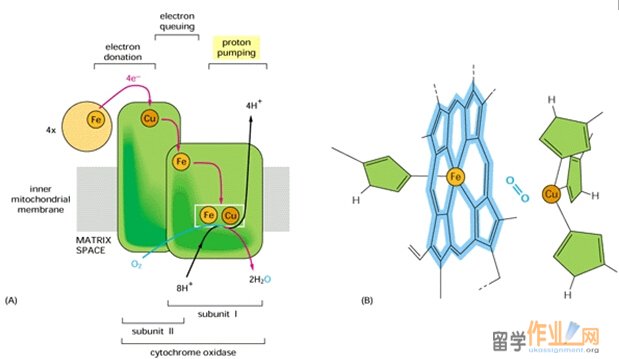 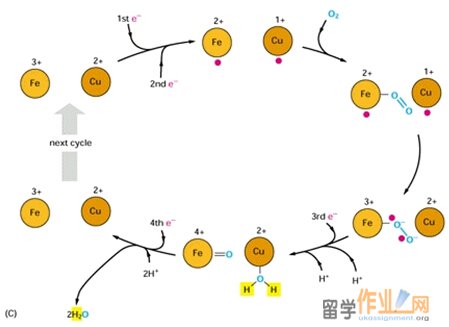
In addition to the four protons used to reduce O2, four protons form the matrix are translocated to the intermembrane space. Thus, for every two electron that traverse complex IV, two protons are translocated. The proposed pathways for proton transfer in complex IV involve hydrogen bond networks of amino acid side chains and water molecules within the protein. In addition, proton pumping in complex IV more closely resembles that of complex I than that of complex III, in which coenzyme Q ferries protons as it undergoes oxidation.
Inhibitors of complex IV:
Cyanide, sulfide, azide, and carbon monoxide all bind to cytochrome oxidase, thus competitively inhibiting the protein from functioning which results in chemical asphyxiation of cells. Methanol is converted into formic acid which also inhibits the same oxidase system.
|
 |
|||
| 网站地图 |

